Home » Lab » FTIR – Fourier-Transform Infrared Spectroscopy
FTIR – Fourier-Transform Infrared Spectroscopy
Thermogravimetric analysis (TGA) measures with very high precision the changes in mass of a material during heating or holding at temperature, providing information on thermal stability and reactions such as solvent loss, oxidation, or degradation up to 1000°C.

Every material in nature possesses a specific chemical structure that distinguishes it: a set of atoms that bond with each other and arrange themselves in space, forming a unique and characteristic order for each type of material.
The FTIR (Fourier-Transform Infrared) spectroscopy technique makes it possible to identify the characteristic vibrations of covalent bonds, which are present in many organic compounds (polymers, resins, coatings, lubricants, etc.) and inorganic compounds (oxides, carbonates, silicates, etc.). A covalent bond is a specific type of very strong chemical bond where two atoms share their (valence) electrons with each other.
In FTIR spectroscopy, an infrared beam is passed through or reflected off the sample to be analysed, and the intensity of the radiation transmitted or absorbed by the material is measured. This technique is based on the principle that each chemical bond absorbs infrared radiation at specific wavelengths, such as C-H, O-H, N-H, C=O, and Si-O. Since each type of compound has a unique arrangement of bonds, the FTIR spectrum can serve as the material’s “fingerprint“.
The FTIR technique is primarily used for the identification of materials (using a database) and the confirmation of materials used in production (both incoming and outgoing). The resulting spectrum is highly specific in most cases, allowing for precise distinction even between similar materials.
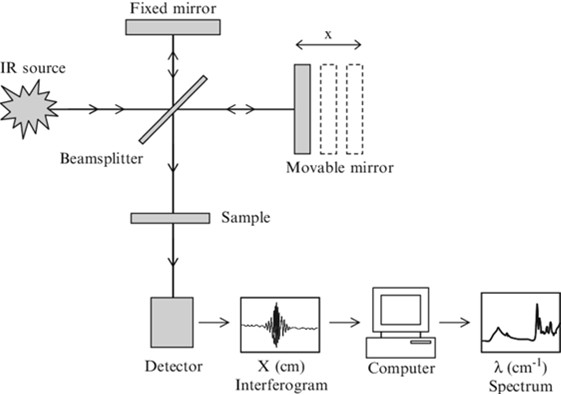
The instrument consists of an IR source, which generates an infrared light beam containing a wide range of wavelengths simultaneously, illuminating the sample. An interferometer, containing a moving mirror, receives the beam after it hits the sample and generates a single signal with all the information (an interferogram). The software then applies a mathematical operator (the Fourier Transform) to the signal and converts it into an interpretable FT-IR spectrum.
FTIR graph example
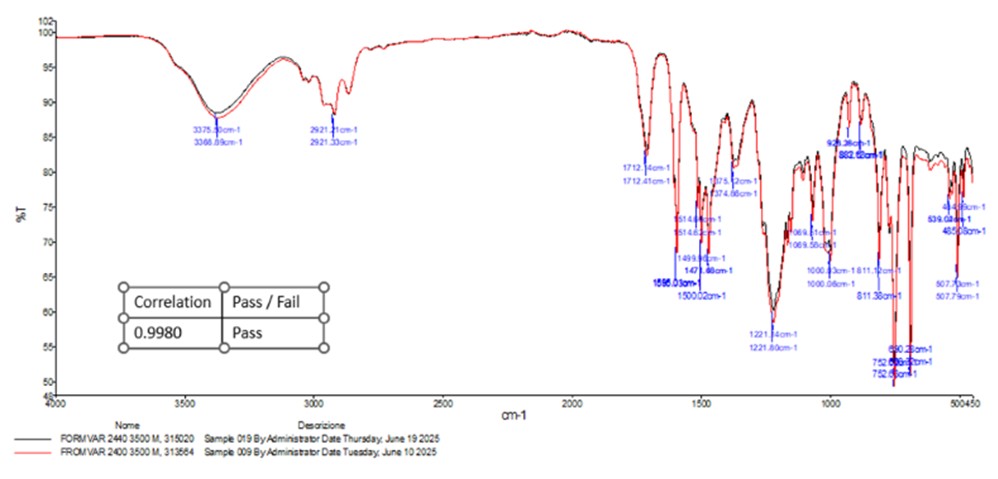
In the example, we see the analysis of spectra obtained from two batches of a Formvar 2440 resin (polyvinyl formal) solution. The spectrum shows peaks corresponding to different absorption bands:
- 3375 cm-1 and 2921 cm-1: O-H and C-H bond stretching;
- 1712 cm-1: C=O bond stretching of ester/aldehyde groups;
- 1506-1600 cm-1: Aromatic C=C bond stretching;
- 1221 cm-1: C-O bond stretching of acetate/ether groups;
- 752-811 cm-1: Aromatic C-H out-of-plane bending.
When comparing two different batches of the same material, the spectra should overlap almost perfectly. In this example, the correlation factor is 0.998, a result that confirms a match in the chemical structure of the two batches, thus passing the quality control check on the material.
Related products
-
CTC – Continuously Transposed Cable
Transposed cable or Continuously Transposed Conductor (CTC) is the most used for windings on power transformers.
Read More → -
ACCM (Aluminium Conductor Composite Multistrand)
Made up of several composite elements in thermo-resistant carbon fiber stranded together it is more flexible and safer than any other conductor
Read More → -
Polyimide FEP Wrapped flat wires
Polyimide-FEP taped wires represent a reliable and advanced answer to the demands of the e-Mobility sector
Read More → -
Peekvest: Extruded PEEK flat wire
The extruded PEEK cable is positioned as a top-of-the-range solution capable of facing the future challenges of the E-Mobility sector
Read More → -
Thervest Enameled flat wire
A wide range of enamelled wires, a product that boasts a thirty-year tradition of production and now adapted to meet automotive needs
Read More →